
We aim to be the nation’s leading manufacturer of generic, affordable pharmaceuticals, enabling both healthcare providers and consumers to benefit from high quality products produced locally at offshore prices. This in turn, reduces costs across the healthcare system while increasing accessibility to healthcare products to patients who need it most without compromising in quality.
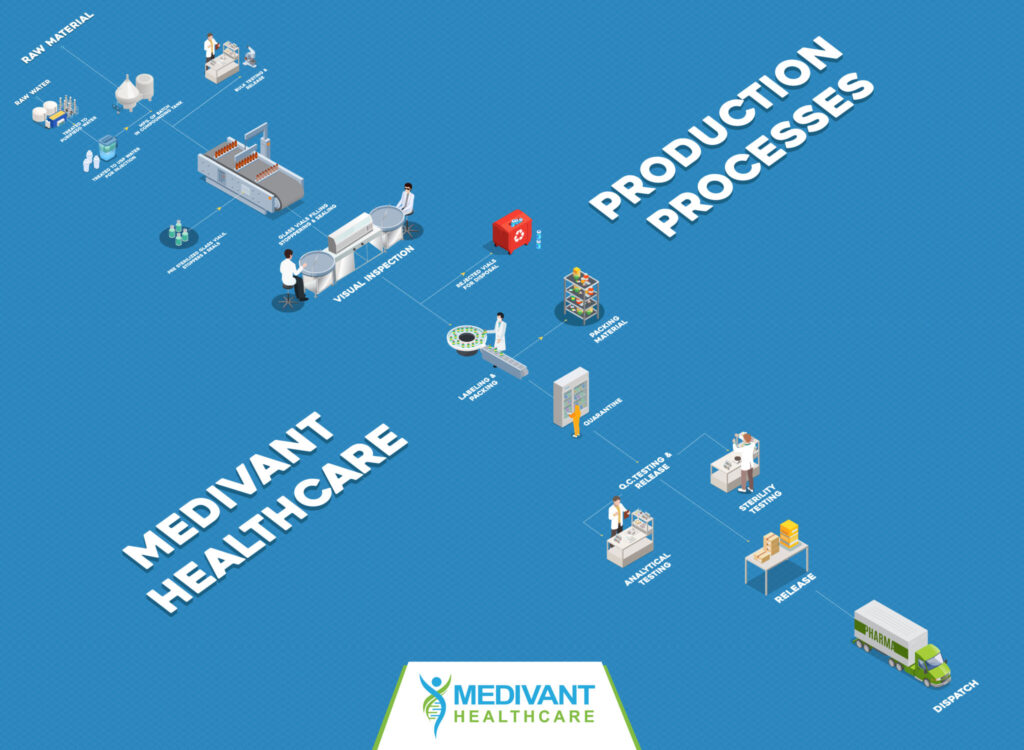
Through our innovative process, we are able to manufacture products from locally procured API that we convert into sterile injections. This significantly decreases costs across the board. We pass along these cost savings to hospitals, healthcare providers, and patients.
Our state-of-the-art, fully automated facility enables us to produce vials at scale. We are investing in research and development to constantly improve our production capability, with the goal of producing 50,000 vials of Single Dose Liquid Injectables (1-10 ml each) per day.
We have maximized efficiency by producing sterile injectables in a single line at our cGMP fully automated plant. Our team of over 20 skilled and comprehensively trained assembly line technicians help manage this process safely and efficiently.
Medivant Healthcare is pioneering the next generation of American manufacturing through our sophisticated, fully automated cGMP manufacturing process. Through our ongoing research and development unit, we are committed to helping patients nationwide by adapting to healthcare needs and providing best in class products, one vial at a time.
Enjoy the next generation of American manufacturing with Medivant Healthcare.
